Climate change units can be confusing. Letters and numbers. Uppercase, lowercase, subscript. How to make sense of a weird agglomeration like GtCO₂e? As long as the article is well written and internally consistent, you may be able to glide right over them and still get the gist. But if you haven’t already, it may be worthwhile to stop a moment and take a closer look, because there’s a lot more hiding in there than might at first appear. Some of it is just quirky. Some of it is really important.
In this post, I’ll focus on the four units that are commonly used when quantifying emissions and greenhouse gases:
- GtCO₂ (gigatonnes of carbon dioxide)
- GtC (gigatonnes of carbon)
- ppm (parts per million)
- GtCO₂e (gigatonnes of carbon dioxide equivalent)
GtCO₂
Let’s start with that first letter, G. That stands for giga, a prefix that means 1 billion, 1,000,000,000 or, in scientific notation, 1×10⁹. You can find a list of other prefixes you may run across here. Weirdly, when you write out ‘gigatonne,’ the g is lowercase. But when you use the abbreviation, as in ‘Gt’ the ‘G’ is always capitalized. Even more weirdly, according to the rules of scientific notation, that rule doesn’t apply to some of the other prefixes, like the ‘k’ in ‘kt’ and kilotonne. That letter is supposed to be lowercase in both instances. Why? Because when the French invented the metric system, they didn’t get higher than one thousand. And also so you can waste time going down this Quora rabbithole.
Next, the lowercase ‘t’. It’s attached to that ‘giga’ and it stands for ‘tonne.’ A ‘tonne’ is the same as a ‘metric ton’ which is 1,000 kilograms, or about 2,205 pounds. (It’s not quite the same as a US ton (2,000 lbs.) or a long or imperial ton (2,240 lbs.), but they’re pretty close. You can probably ignore the differences (unless you are designing a Mars orbiter).1
CO₂, of course, stands for carbon dioxide, so one GtCO₂ is one billion tonnes of CO₂. That’s a lot. So much that it can be hard to wrap your mind around it, even if you know that it is about the same mass as 250 million elephants. At least now, though, the next time you see that unit, you’ll have a strange image in your head of some French elephants about to land on Mars, which should be enough to slow you down.
GtC
Onward to GtC. The ‘C’ in that unit stands for the element carbon. And now we start getting into the confusing stuff. Sometimes scientists prefer to quantify the amount of CO₂ in terms of the carbon alone. For example, instead of seeing: “Globally, the burning of fossil fuels produced 37 GtCO₂,” you might see, “Globally, the burning of fossil fuels produced 10 GtC of CO₂.”
Huh?
Well, in fact, those two statements are saying the same thing. Here’s where the chemistry (and some simple math) comes in. Carbon has an atomic mass of 12.2 The atomic mass of oxygen is 16. That means that the molecular mass of CO₂ is about 44 (12+16+16). And in turn, a carbon dioxide molecule weighs 3.67 (44 ÷ 12) times as much as a carbon atom. Ergo, there are about 10 GtC present in that cloud of 37 GtCO₂ that were released.
The math is pretty easy, but the calculator below will do the work for you if you’d like. Change the number in the top or the bottom box and then press enter. The other box will automatically adjust.
Sometimes climate folks and other scientists prefer to focus on the carbon alone because it’s just easier to follow it through all the transformations that can occur during the so-called carbon cycle. A CO₂ molecule, for example, might be captured by a plant and used to make a sugar molecule (C₆H₁₂O₆). If a cow eats the plant, it might digest the sugar and burp out a methane molecule (CH₄), which might then break down again into carbon dioxide. Instead of doing all the calculations and trying to keep track of all the different molecules, it is sometimes easier just to think in terms of carbon alone.
ppm
Both GtCO₂ and GtC are commonly used to describe emissions. By contrast, ‘ppm’ is commonly used when talking about gases that are already in the atmosphere.
Note that ppm is a unit of concentration. Just as percent stands for ‘parts per hundred,’ ppm stands for ‘parts per million.’ You may also run across ‘ppb’ which stands for parts per billion. You can easily convert between these units. One percent is the same as 10,000 ppm, which is the same as 10,000,000 ppb.
Concentrations like ppm are commonly used when calculating or discussing the greenhouse effect. Why? Because it is not the absolute mass of a greenhouse gas like CO₂ that determines how much energy is trapped by the atmosphere. Rather, it depends on the number of molecules of CO₂ per unit of volume, which is to say concentration.
Now we have covered the first three units: GtCO₂, GtC, and ppm. If you’re a little confused, don’t worry, I promise you’re not the first one. The good news is that it is relatively easy to convert between these three different units. We already covered the conversion between GtC of CO₂ and GtCO₂: 1 GtC of CO₂ = 3.67 GtCO₂.
You can also calculate the conversion from GtC of CO₂ to ppm in the atmosphere if you’d like. You just need to know the number of molecules in a GtC of CO₂ and the total number of all the gas molecules in the atmosphere. In case you don’t want to do that, though, I’ll just tell you. Emitting 2.13 GtC of CO₂ will increase the concentration of CO₂ in the atmosphere by 1 ppm.
Here’s another calculator to play with. Again, if you change the numbers in any of the three fields and press enter, the others will automatically adjust. For example, you will see that increasing the concentration of CO₂ in the atmosphere by 1 ppm is the same as adding 2.13 GtC of CO₂ which is the same as adding 7.81 GtCO₂.
GtCO₂e
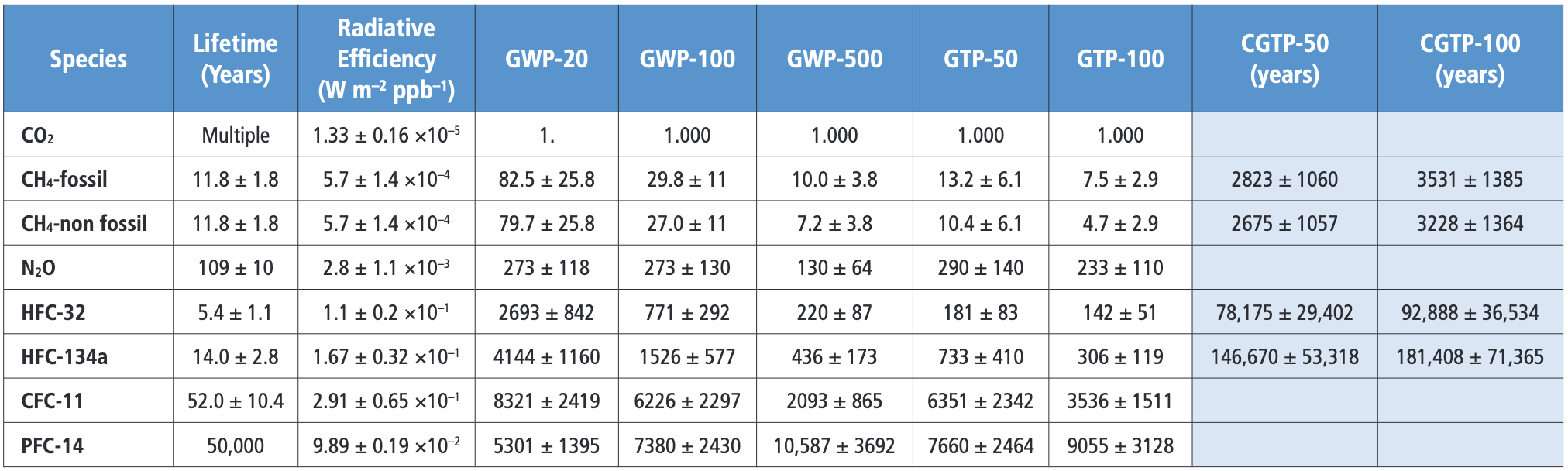
Finally, let’s talk about ‘GtCO₂e.’ That ‘e’ on the end stands for ‘equivalent’ and it comes into play when other greenhouse gases besides CO₂ enter the chat, gases like methane or nitrous oxide (N₂O). At its simplest, GtCO₂e uses CO₂ as a common currency, allowing the comparison and even the trading of the different greenhouse gases. Think of the way the US$ is used in international markets. In this case, though, the exchange rate is based on an estimate of the potency of the different gases over 100 years known as the Global Warming Potential, or GWP-100.
Conceptually, scientifically, and as a matter of policy, GWP-100 is a deeply problematic (and fascinating) metric, but I’ll leave that for another post. For now, just know that the Intergovernmental Panel on Climate Change (IPCC) has assigned every greenhouse gas a GWP-100 value. Methane (CH₄) from fossil fuels, for example, has a GWP-100 of 29.8, meaning that releasing one gigatonne of methane causes as much warming as 29.8 gigatonnes of CO₂.
You’ll run into this GtCO₂e unit anytime the discussion is focused on the aggregate impacts of an activity, organization, or region. For example, in the year 2021, the United States emitted about 6 GtCO₂e of greenhouse gases.
The good news is that once again, the conversions are relatively easy. Here’s another calculator for fossil-fuel methane. Since CH₄ has a molar mass of 16 and carbon has a molar mass of 12, 1 GtC of CH₄ is equal to 1.33 (16 ÷ 12) GtCH₄. You can find the GWP-100 values for other common gases in the IPCC table below.
- In 1999, a US$125 million Mars orbiter crashed after a 10 month journey because one team was using the metric system while another was using British Imperial units. ↩︎
- Yes, I’m simplifying and avoiding using units here! More specifically, 6.02214076×1023 atoms of carbon have a mass of about 12 grams. ↩︎
